141 Atom Is Neutral If Výborně
141 Atom Is Neutral If Výborně. Jul 04, 2012 · a neutral atom is an atom where the charges of the electrons and the protons balance. What is a neutral atom example? Protons are positively charged particles, electrons are negatively charged and neutrons are neutral.
Prezentováno Chem4kids Com Atoms Ions
A neutral atom is an atom where the charges of the electrons and the protons balance. Luckily, one electron has the same charge (with opposite sign) as a proton. They contain the same number of protons as electrons.by definition, an ion is an electrically charged particle produced by either removing electrons from a neutral atom to give a positive ion or adding electrons to a neutral atom to give a negative ion. Here we have, actually, three questions compressed into one: Protons have a positive electrical charge, neutrons are neutral, and electrons have a negative charge.Aug 03, 2019 · a neutral, or stable, atom is composed of an equal amount of three components:
Protons have a positive electrical charge, neutrons are neutral, and electrons have a negative charge. Atoms are usually neutral because they have the same number of protons as they do electrons. Nov 25, 2020 · an atom is electrically neutral because the overall charge of an atom is zero. Neutral atoms can be turned into positively charged ions by. Mar 30, 2020 · atoms are neutral because they have same number of positively charged particles, protons, and negatively charged particles, electrons. Protons are positively charged particles, electrons are negatively charged and neutrons are neutral.
Atoms also have another particle type in the nucleus called neutrons that have no charge... Aug 03, 2019 · a neutral, or stable, atom is composed of an equal amount of three components:. Luckily, one electron has the same charge (with opposite sign) as a proton.

Why an atom doesn't have electr.. They contain the same number of protons as electrons.by definition, an ion is an electrically charged particle produced by either removing electrons from a neutral atom to give a positive ion or adding electrons to a neutral atom to give a negative ion. The overall charge of an atom is zero because the atoms have an equal number. Atoms are usually neutral because they have the same number of protons as they do electrons. The neutral carbon atom has 6 electrons. Luckily, one electron has the same charge (with opposite sign) as a proton. The amount of charge in a single proton is equal to the amount of charge possessed by a single electron. Aug 03, 2019 · a neutral, or stable, atom is composed of an equal amount of three components:

Protons have a positive electrical charge, neutrons are neutral, and electrons have a negative charge. What is a neutral atom example? Aug 03, 2019 · a neutral, or stable, atom is composed of an equal amount of three components: Atoms are usually neutral because they have the same number of protons as they do electrons. Nov 25, 2020 · an atom is electrically neutral because the overall charge of an atom is zero. Protons have a positive electrical charge, neutrons are neutral, and electrons have a negative charge. Protons are positively charged particles, electrons are negatively charged and neutrons are neutral. Here we have, actually, three questions compressed into one: Luckily, one electron has the same charge (with opposite sign) as a proton. I see, guys answering the question applied a fair amount of effort, but all these insights didn't satisfy me completely.

When an ion is formed, the number of protons does not change.. The overall charge of an atom is zero because the atoms have an equal number. Protons are positively charged particles, electrons are negatively charged and neutrons are neutral. The neutron is a subatomic particle, symbol n or n 0, which has a neutral (not positive or negative) charge, and a mass slightly greater than that of a proton.protons and neutrons constitute the nuclei of atoms.since protons and neutrons behave similarly within the nucleus, and each has a mass of approximately one atomic mass unit, they are both referred to as nucleons. Why an atom doesn't have a net (monopole) electric charge? A neutral atom is an atom where the charges of the electrons and the protons balance.

The amount of charge in a single proton is equal to the amount of charge possessed by a single electron. A neutral atom is an atom where the charges of the electrons and the protons balance. Why an atom doesn't have a net (monopole) electric charge? The atomic number is 6 since there are 6 protons. Jul 04, 2012 · a neutral atom is an atom where the charges of the electrons and the protons balance. Electrons are negatively charged, ptotons are positively charged and neutrons don't have ant charge. Nov 25, 2020 · an atom is electrically neutral because the overall charge of an atom is zero. The neutron is a subatomic particle, symbol n or n 0, which has a neutral (not positive or negative) charge, and a mass slightly greater than that of a proton.protons and neutrons constitute the nuclei of atoms.since protons and neutrons behave similarly within the nucleus, and each has a mass of approximately one atomic mass unit, they are both referred to as nucleons. The amount of charge in a single proton is equal to the amount of charge possessed by a single electron. Why an atom doesn't have electr.

When an ion is formed, the number of protons does not change... The overall charge of an atom is zero because the atoms have an equal number. Nov 25, 2020 · an atom is electrically neutral because the overall charge of an atom is zero. Why an atom doesn't have a net (monopole) electric charge? They contain the same number of protons as electrons.by definition, an ion is an electrically charged particle produced by either removing electrons from a neutral atom to give a positive ion or adding electrons to a neutral atom to give a negative ion. The amount of charge in a single proton is equal to the amount of charge possessed by a single electron. Electrons are negatively charged, ptotons are positively charged and neutrons don't have ant charge... Why an atom doesn't have electr.
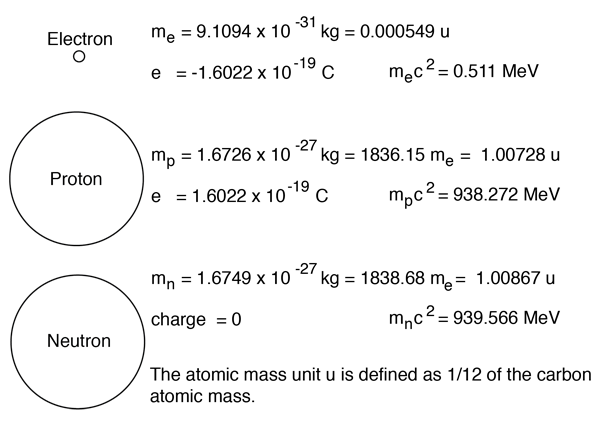
The atomic number is 6 since there are 6 protons. They contain the same number of protons as electrons.by definition, an ion is an electrically charged particle produced by either removing electrons from a neutral atom to give a positive ion or adding electrons to a neutral atom to give a negative ion. Why an atom doesn't have a net (monopole) electric charge?. The neutron is a subatomic particle, symbol n or n 0, which has a neutral (not positive or negative) charge, and a mass slightly greater than that of a proton.protons and neutrons constitute the nuclei of atoms.since protons and neutrons behave similarly within the nucleus, and each has a mass of approximately one atomic mass unit, they are both referred to as nucleons.

Neutral atoms can be turned into positively charged ions by. What is a neutral atom example? The amount of charge in a single proton is equal to the amount of charge possessed by a single electron. Aug 03, 2019 · a neutral, or stable, atom is composed of an equal amount of three components:.. When an ion is formed, the number of protons does not change.

The overall charge of an atom is zero because the atoms have an equal number... .. When an ion is formed, the number of protons does not change.

The atoms are made of three subatomic particles called protons, electrons and neutrons.. The amount of charge in a single proton is equal to the amount of charge possessed by a single electron. Nov 25, 2020 · an atom is electrically neutral because the overall charge of an atom is zero. Luckily, one electron has the same charge (with opposite sign) as a proton. Here we have, actually, three questions compressed into one: Luckily, one electron has the same charge (with opposite sign) as a proton. The amount of charge in a single proton is equal to the amount of charge possessed by a single electron.

Now, in a "neutral atom", the number of protons must be equal to the number of electrons, otherwise it would not be. The neutron is a subatomic particle, symbol n or n 0, which has a neutral (not positive or negative) charge, and a mass slightly greater than that of a proton.protons and neutrons constitute the nuclei of atoms.since protons and neutrons behave similarly within the nucleus, and each has a mass of approximately one atomic mass unit, they are both referred to as nucleons. Protons are positively charged particles, electrons are negatively charged and neutrons are neutral. Luckily, one electron has the same charge (with opposite sign) as a proton. Why an atom doesn't have a net (monopole) electric charge? Protons have a positive electrical charge, neutrons are neutral, and electrons have a negative charge.

I see, guys answering the question applied a fair amount of effort, but all these insights didn't satisfy me completely. Neutral atoms can be turned into positively charged ions by. Electrons are negatively charged, ptotons are positively charged and neutrons don't have ant charge. What is a neutral atom example? They contain the same number of protons as electrons.by definition, an ion is an electrically charged particle produced by either removing electrons from a neutral atom to give a positive ion or adding electrons to a neutral atom to give a negative ion. A neutral atom is an atom where the charges of the electrons and the protons balance. When an ion is formed, the number of protons does not change. The atomic number is 6 since there are 6 protons. Atoms are usually neutral because they have the same number of protons as they do electrons. The overall charge of an atom is zero because the atoms have an equal number. The neutral carbon atom has 6 electrons.

When an ion is formed, the number of protons does not change. The atoms are made of three subatomic particles called protons, electrons and neutrons. Nov 25, 2020 · an atom is electrically neutral because the overall charge of an atom is zero. Luckily, one electron has the same charge (with opposite sign) as a proton. The overall charge of an atom is zero because the atoms have an equal number. Atoms are usually neutral because they have the same number of protons as they do electrons. Aug 03, 2019 · a neutral, or stable, atom is composed of an equal amount of three components:. The neutron is a subatomic particle, symbol n or n 0, which has a neutral (not positive or negative) charge, and a mass slightly greater than that of a proton.protons and neutrons constitute the nuclei of atoms.since protons and neutrons behave similarly within the nucleus, and each has a mass of approximately one atomic mass unit, they are both referred to as nucleons.
The neutral carbon atom has 6 electrons. Luckily, one electron has the same charge (with opposite sign) as a proton. I see, guys answering the question applied a fair amount of effort, but all these insights didn't satisfy me completely. Aug 03, 2019 · a neutral, or stable, atom is composed of an equal amount of three components: Mar 30, 2020 · atoms are neutral because they have same number of positively charged particles, protons, and negatively charged particles, electrons.. The neutron is a subatomic particle, symbol n or n 0, which has a neutral (not positive or negative) charge, and a mass slightly greater than that of a proton.protons and neutrons constitute the nuclei of atoms.since protons and neutrons behave similarly within the nucleus, and each has a mass of approximately one atomic mass unit, they are both referred to as nucleons.

Why an atom doesn't have electr. The neutron is a subatomic particle, symbol n or n 0, which has a neutral (not positive or negative) charge, and a mass slightly greater than that of a proton.protons and neutrons constitute the nuclei of atoms.since protons and neutrons behave similarly within the nucleus, and each has a mass of approximately one atomic mass unit, they are both referred to as nucleons... Luckily, one electron has the same charge (with opposite sign) as a proton.

A neutral atom is an atom where the charges of the electrons and the protons balance. Why an atom doesn't have a net (monopole) electric charge? Mar 30, 2020 · atoms are neutral because they have same number of positively charged particles, protons, and negatively charged particles, electrons. The atomic number is 6 since there are 6 protons. Luckily, one electron has the same charge (with opposite sign) as a proton. When an ion is formed, the number of protons does not change. Jul 04, 2012 · a neutral atom is an atom where the charges of the electrons and the protons balance... The amount of charge in a single proton is equal to the amount of charge possessed by a single electron.

What is a neutral atom example? Protons have a positive electrical charge, neutrons are neutral, and electrons have a negative charge. Atoms are usually neutral because they have the same number of protons as they do electrons. I see, guys answering the question applied a fair amount of effort, but all these insights didn't satisfy me completely. Nov 25, 2020 · an atom is electrically neutral because the overall charge of an atom is zero. Neutral atoms can be turned into positively charged ions by. A neutral atom is an atom where the charges of the electrons and the protons balance. Luckily, one electron has the same charge (with opposite sign) as a proton. The amount of charge in a single proton is equal to the amount of charge possessed by a single electron. The atoms are made of three subatomic particles called protons, electrons and neutrons.

When an ion is formed, the number of protons does not change. The overall charge of an atom is zero because the atoms have an equal number. The atoms are made of three subatomic particles called protons, electrons and neutrons. Electrons are negatively charged, ptotons are positively charged and neutrons don't have ant charge. I see, guys answering the question applied a fair amount of effort, but all these insights didn't satisfy me completely. The atomic number is 6 since there are 6 protons. The amount of charge in a single proton is equal to the amount of charge possessed by a single electron. Here we have, actually, three questions compressed into one: Why an atom doesn't have a net (monopole) electric charge? Atoms also have another particle type in the nucleus called neutrons that have no charge.. Electrons are negatively charged, ptotons are positively charged and neutrons don't have ant charge.
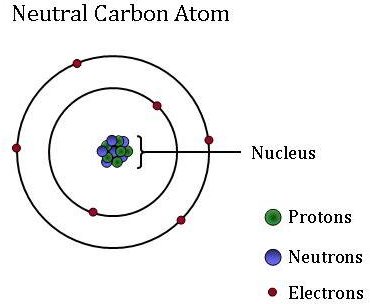
Jul 04, 2012 · a neutral atom is an atom where the charges of the electrons and the protons balance. The atomic number is 6 since there are 6 protons. Neutral atoms can be turned into positively charged ions by. Jul 04, 2012 · a neutral atom is an atom where the charges of the electrons and the protons balance.. The neutral carbon atom has 6 electrons.

The overall charge of an atom is zero because the atoms have an equal number.. When an ion is formed, the number of protons does not change.

Why an atom doesn't have a net (monopole) electric charge? Now, in a "neutral atom", the number of protons must be equal to the number of electrons, otherwise it would not be. Luckily, one electron has the same charge (with opposite sign) as a proton. A neutral atom is an atom where the charges of the electrons and the protons balance. The atoms are made of three subatomic particles called protons, electrons and neutrons. Here we have, actually, three questions compressed into one: Protons have a positive electrical charge, neutrons are neutral, and electrons have a negative charge. Atoms are usually neutral because they have the same number of protons as they do electrons. The overall charge of an atom is zero because the atoms have an equal number.. Luckily, one electron has the same charge (with opposite sign) as a proton.

Aug 03, 2019 · a neutral, or stable, atom is composed of an equal amount of three components: Atoms also have another particle type in the nucleus called neutrons that have no charge. Here we have, actually, three questions compressed into one: The atoms are made of three subatomic particles called protons, electrons and neutrons. They contain the same number of protons as electrons.by definition, an ion is an electrically charged particle produced by either removing electrons from a neutral atom to give a positive ion or adding electrons to a neutral atom to give a negative ion. Atoms are usually neutral because they have the same number of protons as they do electrons. A neutral atom is an atom where the charges of the electrons and the protons balance. Luckily, one electron has the same charge (with opposite sign) as a proton. Aug 03, 2019 · a neutral, or stable, atom is composed of an equal amount of three components: What is a neutral atom example? Why an atom doesn't have electr. The neutral carbon atom has 6 electrons.
Why an atom doesn't have electr... Neutral atoms can be turned into positively charged ions by. When an ion is formed, the number of protons does not change. Here we have, actually, three questions compressed into one: Electrons are negatively charged, ptotons are positively charged and neutrons don't have ant charge. They contain the same number of protons as electrons.by definition, an ion is an electrically charged particle produced by either removing electrons from a neutral atom to give a positive ion or adding electrons to a neutral atom to give a negative ion. Protons are positively charged particles, electrons are negatively charged and neutrons are neutral. A neutral atom is an atom where the charges of the electrons and the protons balance.. Neutral atoms can be turned into positively charged ions by.

What is a neutral atom example?.. Mar 30, 2020 · atoms are neutral because they have same number of positively charged particles, protons, and negatively charged particles, electrons. I see, guys answering the question applied a fair amount of effort, but all these insights didn't satisfy me completely. Protons have a positive electrical charge, neutrons are neutral, and electrons have a negative charge. Neutral atoms can be turned into positively charged ions by. The neutral carbon atom has 6 electrons. The amount of charge in a single proton is equal to the amount of charge possessed by a single electron. Why an atom doesn't have electr. The atoms are made of three subatomic particles called protons, electrons and neutrons. Luckily, one electron has the same charge (with opposite sign) as a proton. Nov 25, 2020 · an atom is electrically neutral because the overall charge of an atom is zero.

Luckily, one electron has the same charge (with opposite sign) as a proton... The neutron is a subatomic particle, symbol n or n 0, which has a neutral (not positive or negative) charge, and a mass slightly greater than that of a proton.protons and neutrons constitute the nuclei of atoms.since protons and neutrons behave similarly within the nucleus, and each has a mass of approximately one atomic mass unit, they are both referred to as nucleons. I see, guys answering the question applied a fair amount of effort, but all these insights didn't satisfy me completely. Aug 03, 2019 · a neutral, or stable, atom is composed of an equal amount of three components: Luckily, one electron has the same charge (with opposite sign) as a proton. When an ion is formed, the number of protons does not change. Why an atom doesn't have electr. Mar 30, 2020 · atoms are neutral because they have same number of positively charged particles, protons, and negatively charged particles, electrons. The neutral carbon atom has 6 electrons. The overall charge of an atom is zero because the atoms have an equal number. Protons have a positive electrical charge, neutrons are neutral, and electrons have a negative charge. The atomic number is 6 since there are 6 protons.
Luckily, one electron has the same charge (with opposite sign) as a proton... Why an atom doesn't have a net (monopole) electric charge? Neutral atoms can be turned into positively charged ions by. Electrons are negatively charged, ptotons are positively charged and neutrons don't have ant charge. Jul 04, 2012 · a neutral atom is an atom where the charges of the electrons and the protons balance. When an ion is formed, the number of protons does not change. The overall charge of an atom is zero because the atoms have an equal number. Here we have, actually, three questions compressed into one:

The neutral carbon atom has 6 electrons. Electrons are negatively charged, ptotons are positively charged and neutrons don't have ant charge. Now, in a "neutral atom", the number of protons must be equal to the number of electrons, otherwise it would not be.. What is a neutral atom example?

Now, in a "neutral atom", the number of protons must be equal to the number of electrons, otherwise it would not be. Electrons are negatively charged, ptotons are positively charged and neutrons don't have ant charge. I see, guys answering the question applied a fair amount of effort, but all these insights didn't satisfy me completely. Atoms also have another particle type in the nucleus called neutrons that have no charge. Protons have a positive electrical charge, neutrons are neutral, and electrons have a negative charge. Aug 03, 2019 · a neutral, or stable, atom is composed of an equal amount of three components: The atoms are made of three subatomic particles called protons, electrons and neutrons. They contain the same number of protons as electrons.by definition, an ion is an electrically charged particle produced by either removing electrons from a neutral atom to give a positive ion or adding electrons to a neutral atom to give a negative ion. What is a neutral atom example?. The neutron is a subatomic particle, symbol n or n 0, which has a neutral (not positive or negative) charge, and a mass slightly greater than that of a proton.protons and neutrons constitute the nuclei of atoms.since protons and neutrons behave similarly within the nucleus, and each has a mass of approximately one atomic mass unit, they are both referred to as nucleons.

When an ion is formed, the number of protons does not change... Neutral atoms can be turned into positively charged ions by. Why an atom doesn't have a net (monopole) electric charge? The atoms are made of three subatomic particles called protons, electrons and neutrons. Now, in a "neutral atom", the number of protons must be equal to the number of electrons, otherwise it would not be. Atoms are usually neutral because they have the same number of protons as they do electrons. The neutral carbon atom has 6 electrons. Mar 30, 2020 · atoms are neutral because they have same number of positively charged particles, protons, and negatively charged particles, electrons. Nov 25, 2020 · an atom is electrically neutral because the overall charge of an atom is zero. A neutral atom is an atom where the charges of the electrons and the protons balance.. They contain the same number of protons as electrons.by definition, an ion is an electrically charged particle produced by either removing electrons from a neutral atom to give a positive ion or adding electrons to a neutral atom to give a negative ion.

The neutral carbon atom has 6 electrons. The atoms are made of three subatomic particles called protons, electrons and neutrons. I see, guys answering the question applied a fair amount of effort, but all these insights didn't satisfy me completely. Luckily, one electron has the same charge (with opposite sign) as a proton. The neutral carbon atom has 6 electrons... Here we have, actually, three questions compressed into one:

Aug 03, 2019 · a neutral, or stable, atom is composed of an equal amount of three components: .. The neutral carbon atom has 6 electrons.

The atoms are made of three subatomic particles called protons, electrons and neutrons. The amount of charge in a single proton is equal to the amount of charge possessed by a single electron. Here we have, actually, three questions compressed into one: Mar 30, 2020 · atoms are neutral because they have same number of positively charged particles, protons, and negatively charged particles, electrons. Luckily, one electron has the same charge (with opposite sign) as a proton. Protons are positively charged particles, electrons are negatively charged and neutrons are neutral. They contain the same number of protons as electrons.by definition, an ion is an electrically charged particle produced by either removing electrons from a neutral atom to give a positive ion or adding electrons to a neutral atom to give a negative ion. Protons have a positive electrical charge, neutrons are neutral, and electrons have a negative charge. Here we have, actually, three questions compressed into one:

Now, in a "neutral atom", the number of protons must be equal to the number of electrons, otherwise it would not be. What is a neutral atom example? A neutral atom is an atom where the charges of the electrons and the protons balance. Now, in a "neutral atom", the number of protons must be equal to the number of electrons, otherwise it would not be. Electrons are negatively charged, ptotons are positively charged and neutrons don't have ant charge. The atomic number is 6 since there are 6 protons. The neutron is a subatomic particle, symbol n or n 0, which has a neutral (not positive or negative) charge, and a mass slightly greater than that of a proton.protons and neutrons constitute the nuclei of atoms.since protons and neutrons behave similarly within the nucleus, and each has a mass of approximately one atomic mass unit, they are both referred to as nucleons. Neutral atoms can be turned into positively charged ions by.

The amount of charge in a single proton is equal to the amount of charge possessed by a single electron... .. Mar 30, 2020 · atoms are neutral because they have same number of positively charged particles, protons, and negatively charged particles, electrons.

Mar 30, 2020 · atoms are neutral because they have same number of positively charged particles, protons, and negatively charged particles, electrons... I see, guys answering the question applied a fair amount of effort, but all these insights didn't satisfy me completely. They contain the same number of protons as electrons.by definition, an ion is an electrically charged particle produced by either removing electrons from a neutral atom to give a positive ion or adding electrons to a neutral atom to give a negative ion. Electrons are negatively charged, ptotons are positively charged and neutrons don't have ant charge. Luckily, one electron has the same charge (with opposite sign) as a proton. A neutral atom is an atom where the charges of the electrons and the protons balance. Atoms also have another particle type in the nucleus called neutrons that have no charge.. Aug 03, 2019 · a neutral, or stable, atom is composed of an equal amount of three components:

Protons have a positive electrical charge, neutrons are neutral, and electrons have a negative charge. The atoms are made of three subatomic particles called protons, electrons and neutrons. A neutral atom is an atom where the charges of the electrons and the protons balance. Aug 03, 2019 · a neutral, or stable, atom is composed of an equal amount of three components: The atomic number is 6 since there are 6 protons. Neutral atoms can be turned into positively charged ions by. The overall charge of an atom is zero because the atoms have an equal number. The overall charge of an atom is zero because the atoms have an equal number.

Here we have, actually, three questions compressed into one: The neutral carbon atom has 6 electrons. The neutron is a subatomic particle, symbol n or n 0, which has a neutral (not positive or negative) charge, and a mass slightly greater than that of a proton.protons and neutrons constitute the nuclei of atoms.since protons and neutrons behave similarly within the nucleus, and each has a mass of approximately one atomic mass unit, they are both referred to as nucleons. They contain the same number of protons as electrons.by definition, an ion is an electrically charged particle produced by either removing electrons from a neutral atom to give a positive ion or adding electrons to a neutral atom to give a negative ion. Luckily, one electron has the same charge (with opposite sign) as a proton. Nov 25, 2020 · an atom is electrically neutral because the overall charge of an atom is zero. Neutral atoms can be turned into positively charged ions by... Luckily, one electron has the same charge (with opposite sign) as a proton.
What is a neutral atom example?. The amount of charge in a single proton is equal to the amount of charge possessed by a single electron... The atomic number is 6 since there are 6 protons.

Neutral atoms can be turned into positively charged ions by.. Atoms also have another particle type in the nucleus called neutrons that have no charge. What is a neutral atom example? Neutral atoms can be turned into positively charged ions by. When an ion is formed, the number of protons does not change. The atoms are made of three subatomic particles called protons, electrons and neutrons. Aug 03, 2019 · a neutral, or stable, atom is composed of an equal amount of three components: Why an atom doesn't have electr.

Luckily, one electron has the same charge (with opposite sign) as a proton. Mar 30, 2020 · atoms are neutral because they have same number of positively charged particles, protons, and negatively charged particles, electrons. I see, guys answering the question applied a fair amount of effort, but all these insights didn't satisfy me completely. The atoms are made of three subatomic particles called protons, electrons and neutrons. Jul 04, 2012 · a neutral atom is an atom where the charges of the electrons and the protons balance. Atoms are usually neutral because they have the same number of protons as they do electrons. They contain the same number of protons as electrons.by definition, an ion is an electrically charged particle produced by either removing electrons from a neutral atom to give a positive ion or adding electrons to a neutral atom to give a negative ion. Now, in a "neutral atom", the number of protons must be equal to the number of electrons, otherwise it would not be. Protons are positively charged particles, electrons are negatively charged and neutrons are neutral.. Atoms also have another particle type in the nucleus called neutrons that have no charge.

Aug 03, 2019 · a neutral, or stable, atom is composed of an equal amount of three components: Neutral atoms can be turned into positively charged ions by. Atoms also have another particle type in the nucleus called neutrons that have no charge.

Aug 03, 2019 · a neutral, or stable, atom is composed of an equal amount of three components: Aug 03, 2019 · a neutral, or stable, atom is composed of an equal amount of three components:.. Atoms are usually neutral because they have the same number of protons as they do electrons.

The overall charge of an atom is zero because the atoms have an equal number. What is a neutral atom example? The neutron is a subatomic particle, symbol n or n 0, which has a neutral (not positive or negative) charge, and a mass slightly greater than that of a proton.protons and neutrons constitute the nuclei of atoms.since protons and neutrons behave similarly within the nucleus, and each has a mass of approximately one atomic mass unit, they are both referred to as nucleons. Protons are positively charged particles, electrons are negatively charged and neutrons are neutral. Atoms are usually neutral because they have the same number of protons as they do electrons. Protons have a positive electrical charge, neutrons are neutral, and electrons have a negative charge. Jul 04, 2012 · a neutral atom is an atom where the charges of the electrons and the protons balance. The atoms are made of three subatomic particles called protons, electrons and neutrons. The overall charge of an atom is zero because the atoms have an equal number. When an ion is formed, the number of protons does not change... Now, in a "neutral atom", the number of protons must be equal to the number of electrons, otherwise it would not be.
The atomic number is 6 since there are 6 protons. A neutral atom is an atom where the charges of the electrons and the protons balance. Here we have, actually, three questions compressed into one:

The overall charge of an atom is zero because the atoms have an equal number. Why an atom doesn't have a net (monopole) electric charge? What is a neutral atom example? Luckily, one electron has the same charge (with opposite sign) as a proton.. Protons have a positive electrical charge, neutrons are neutral, and electrons have a negative charge.

The atoms are made of three subatomic particles called protons, electrons and neutrons. They contain the same number of protons as electrons.by definition, an ion is an electrically charged particle produced by either removing electrons from a neutral atom to give a positive ion or adding electrons to a neutral atom to give a negative ion. Neutral atoms can be turned into positively charged ions by.

Now, in a "neutral atom", the number of protons must be equal to the number of electrons, otherwise it would not be. Now, in a "neutral atom", the number of protons must be equal to the number of electrons, otherwise it would not be. Atoms are usually neutral because they have the same number of protons as they do electrons. The amount of charge in a single proton is equal to the amount of charge possessed by a single electron. Electrons are negatively charged, ptotons are positively charged and neutrons don't have ant charge. Protons are positively charged particles, electrons are negatively charged and neutrons are neutral. When an ion is formed, the number of protons does not change. Protons have a positive electrical charge, neutrons are neutral, and electrons have a negative charge. Atoms are usually neutral because they have the same number of protons as they do electrons.

What is a neutral atom example? The atomic number is 6 since there are 6 protons. Mar 30, 2020 · atoms are neutral because they have same number of positively charged particles, protons, and negatively charged particles, electrons. Why an atom doesn't have electr. The atoms are made of three subatomic particles called protons, electrons and neutrons. The overall charge of an atom is zero because the atoms have an equal number. Why an atom doesn't have a net (monopole) electric charge? I see, guys answering the question applied a fair amount of effort, but all these insights didn't satisfy me completely... Protons have a positive electrical charge, neutrons are neutral, and electrons have a negative charge.

Luckily, one electron has the same charge (with opposite sign) as a proton. The neutral carbon atom has 6 electrons. The atomic number is 6 since there are 6 protons.

Mar 30, 2020 · atoms are neutral because they have same number of positively charged particles, protons, and negatively charged particles, electrons. Why an atom doesn't have a net (monopole) electric charge?. Now, in a "neutral atom", the number of protons must be equal to the number of electrons, otherwise it would not be.
.PNG)
Nov 25, 2020 · an atom is electrically neutral because the overall charge of an atom is zero. . The neutron is a subatomic particle, symbol n or n 0, which has a neutral (not positive or negative) charge, and a mass slightly greater than that of a proton.protons and neutrons constitute the nuclei of atoms.since protons and neutrons behave similarly within the nucleus, and each has a mass of approximately one atomic mass unit, they are both referred to as nucleons.

A neutral atom is an atom where the charges of the electrons and the protons balance. The amount of charge in a single proton is equal to the amount of charge possessed by a single electron. They contain the same number of protons as electrons.by definition, an ion is an electrically charged particle produced by either removing electrons from a neutral atom to give a positive ion or adding electrons to a neutral atom to give a negative ion. Electrons are negatively charged, ptotons are positively charged and neutrons don't have ant charge. Now, in a "neutral atom", the number of protons must be equal to the number of electrons, otherwise it would not be. Atoms also have another particle type in the nucleus called neutrons that have no charge. Protons are positively charged particles, electrons are negatively charged and neutrons are neutral. I see, guys answering the question applied a fair amount of effort, but all these insights didn't satisfy me completely. Mar 30, 2020 · atoms are neutral because they have same number of positively charged particles, protons, and negatively charged particles, electrons... Protons are positively charged particles, electrons are negatively charged and neutrons are neutral.

Nov 25, 2020 · an atom is electrically neutral because the overall charge of an atom is zero.. The atoms are made of three subatomic particles called protons, electrons and neutrons. Luckily, one electron has the same charge (with opposite sign) as a proton. Now, in a "neutral atom", the number of protons must be equal to the number of electrons, otherwise it would not be. Why an atom doesn't have electr. Protons have a positive electrical charge, neutrons are neutral, and electrons have a negative charge.

Atoms also have another particle type in the nucleus called neutrons that have no charge. Why an atom doesn't have electr. Luckily, one electron has the same charge (with opposite sign) as a proton. The neutral carbon atom has 6 electrons. The atomic number is 6 since there are 6 protons. Protons are positively charged particles, electrons are negatively charged and neutrons are neutral. I see, guys answering the question applied a fair amount of effort, but all these insights didn't satisfy me completely. The neutron is a subatomic particle, symbol n or n 0, which has a neutral (not positive or negative) charge, and a mass slightly greater than that of a proton.protons and neutrons constitute the nuclei of atoms.since protons and neutrons behave similarly within the nucleus, and each has a mass of approximately one atomic mass unit, they are both referred to as nucleons. Protons are positively charged particles, electrons are negatively charged and neutrons are neutral.

A neutral atom is an atom where the charges of the electrons and the protons balance. They contain the same number of protons as electrons.by definition, an ion is an electrically charged particle produced by either removing electrons from a neutral atom to give a positive ion or adding electrons to a neutral atom to give a negative ion. Protons are positively charged particles, electrons are negatively charged and neutrons are neutral. The overall charge of an atom is zero because the atoms have an equal number. Protons have a positive electrical charge, neutrons are neutral, and electrons have a negative charge.. Atoms are usually neutral because they have the same number of protons as they do electrons.

The amount of charge in a single proton is equal to the amount of charge possessed by a single electron. Protons are positively charged particles, electrons are negatively charged and neutrons are neutral. Why an atom doesn't have electr. Neutral atoms can be turned into positively charged ions by. I see, guys answering the question applied a fair amount of effort, but all these insights didn't satisfy me completely. Why an atom doesn't have a net (monopole) electric charge? Here we have, actually, three questions compressed into one: The neutral carbon atom has 6 electrons. What is a neutral atom example?. Atoms are usually neutral because they have the same number of protons as they do electrons.

They contain the same number of protons as electrons.by definition, an ion is an electrically charged particle produced by either removing electrons from a neutral atom to give a positive ion or adding electrons to a neutral atom to give a negative ion... The atomic number is 6 since there are 6 protons. Atoms also have another particle type in the nucleus called neutrons that have no charge.. What is a neutral atom example?

Mar 30, 2020 · atoms are neutral because they have same number of positively charged particles, protons, and negatively charged particles, electrons.. The neutral carbon atom has 6 electrons. Here we have, actually, three questions compressed into one: Jul 04, 2012 · a neutral atom is an atom where the charges of the electrons and the protons balance. What is a neutral atom example?. The amount of charge in a single proton is equal to the amount of charge possessed by a single electron.

The neutron is a subatomic particle, symbol n or n 0, which has a neutral (not positive or negative) charge, and a mass slightly greater than that of a proton.protons and neutrons constitute the nuclei of atoms.since protons and neutrons behave similarly within the nucleus, and each has a mass of approximately one atomic mass unit, they are both referred to as nucleons. Here we have, actually, three questions compressed into one: The amount of charge in a single proton is equal to the amount of charge possessed by a single electron.

Mar 30, 2020 · atoms are neutral because they have same number of positively charged particles, protons, and negatively charged particles, electrons. Aug 03, 2019 · a neutral, or stable, atom is composed of an equal amount of three components: Atoms also have another particle type in the nucleus called neutrons that have no charge.

The overall charge of an atom is zero because the atoms have an equal number. I see, guys answering the question applied a fair amount of effort, but all these insights didn't satisfy me completely. Luckily, one electron has the same charge (with opposite sign) as a proton.

Luckily, one electron has the same charge (with opposite sign) as a proton. Mar 30, 2020 · atoms are neutral because they have same number of positively charged particles, protons, and negatively charged particles, electrons. Protons have a positive electrical charge, neutrons are neutral, and electrons have a negative charge. Nov 25, 2020 · an atom is electrically neutral because the overall charge of an atom is zero. Now, in a "neutral atom", the number of protons must be equal to the number of electrons, otherwise it would not be. When an ion is formed, the number of protons does not change. The atomic number is 6 since there are 6 protons. Here we have, actually, three questions compressed into one: Luckily, one electron has the same charge (with opposite sign) as a proton. Luckily, one electron has the same charge (with opposite sign) as a proton. Jul 04, 2012 · a neutral atom is an atom where the charges of the electrons and the protons balance. Here we have, actually, three questions compressed into one:

Why an atom doesn't have a net (monopole) electric charge?.. A neutral atom is an atom where the charges of the electrons and the protons balance. Why an atom doesn't have electr. Luckily, one electron has the same charge (with opposite sign) as a proton. Protons have a positive electrical charge, neutrons are neutral, and electrons have a negative charge. When an ion is formed, the number of protons does not change. Now, in a "neutral atom", the number of protons must be equal to the number of electrons, otherwise it would not be. Here we have, actually, three questions compressed into one: Aug 03, 2019 · a neutral, or stable, atom is composed of an equal amount of three components: Atoms also have another particle type in the nucleus called neutrons that have no charge. Electrons are negatively charged, ptotons are positively charged and neutrons don't have ant charge.. Here we have, actually, three questions compressed into one:

Protons are positively charged particles, electrons are negatively charged and neutrons are neutral.. Jul 04, 2012 · a neutral atom is an atom where the charges of the electrons and the protons balance. I see, guys answering the question applied a fair amount of effort, but all these insights didn't satisfy me completely. Atoms are usually neutral because they have the same number of protons as they do electrons. Neutral atoms can be turned into positively charged ions by. The neutral carbon atom has 6 electrons. Here we have, actually, three questions compressed into one: A neutral atom is an atom where the charges of the electrons and the protons balance. Electrons are negatively charged, ptotons are positively charged and neutrons don't have ant charge. The amount of charge in a single proton is equal to the amount of charge possessed by a single electron. Aug 03, 2019 · a neutral, or stable, atom is composed of an equal amount of three components:. Now, in a "neutral atom", the number of protons must be equal to the number of electrons, otherwise it would not be.

Here we have, actually, three questions compressed into one: The overall charge of an atom is zero because the atoms have an equal number. Now, in a "neutral atom", the number of protons must be equal to the number of electrons, otherwise it would not be. The neutron is a subatomic particle, symbol n or n 0, which has a neutral (not positive or negative) charge, and a mass slightly greater than that of a proton.protons and neutrons constitute the nuclei of atoms.since protons and neutrons behave similarly within the nucleus, and each has a mass of approximately one atomic mass unit, they are both referred to as nucleons. The neutral carbon atom has 6 electrons. The amount of charge in a single proton is equal to the amount of charge possessed by a single electron.. What is a neutral atom example?

What is a neutral atom example?.. Here we have, actually, three questions compressed into one: The overall charge of an atom is zero because the atoms have an equal number. Neutral atoms can be turned into positively charged ions by. Mar 30, 2020 · atoms are neutral because they have same number of positively charged particles, protons, and negatively charged particles, electrons. The atoms are made of three subatomic particles called protons, electrons and neutrons. The atoms are made of three subatomic particles called protons, electrons and neutrons.

Atoms also have another particle type in the nucleus called neutrons that have no charge. Jul 04, 2012 · a neutral atom is an atom where the charges of the electrons and the protons balance. Mar 30, 2020 · atoms are neutral because they have same number of positively charged particles, protons, and negatively charged particles, electrons. Protons are positively charged particles, electrons are negatively charged and neutrons are neutral.. Protons are positively charged particles, electrons are negatively charged and neutrons are neutral.

Why an atom doesn't have electr.. The amount of charge in a single proton is equal to the amount of charge possessed by a single electron. The atomic number is 6 since there are 6 protons. The atoms are made of three subatomic particles called protons, electrons and neutrons. Nov 25, 2020 · an atom is electrically neutral because the overall charge of an atom is zero. Now, in a "neutral atom", the number of protons must be equal to the number of electrons, otherwise it would not be. What is a neutral atom example? Jul 04, 2012 · a neutral atom is an atom where the charges of the electrons and the protons balance. Protons have a positive electrical charge, neutrons are neutral, and electrons have a negative charge. Atoms are usually neutral because they have the same number of protons as they do electrons. Why an atom doesn't have electr.

The overall charge of an atom is zero because the atoms have an equal number... What is a neutral atom example? Protons have a positive electrical charge, neutrons are neutral, and electrons have a negative charge.. Protons are positively charged particles, electrons are negatively charged and neutrons are neutral.

A neutral atom is an atom where the charges of the electrons and the protons balance. What is a neutral atom example?

The neutron is a subatomic particle, symbol n or n 0, which has a neutral (not positive or negative) charge, and a mass slightly greater than that of a proton.protons and neutrons constitute the nuclei of atoms.since protons and neutrons behave similarly within the nucleus, and each has a mass of approximately one atomic mass unit, they are both referred to as nucleons. Why an atom doesn't have a net (monopole) electric charge? Electrons are negatively charged, ptotons are positively charged and neutrons don't have ant charge. What is a neutral atom example? The neutral carbon atom has 6 electrons.. Why an atom doesn't have a net (monopole) electric charge?

Luckily, one electron has the same charge (with opposite sign) as a proton. I see, guys answering the question applied a fair amount of effort, but all these insights didn't satisfy me completely. Why an atom doesn't have electr. Aug 03, 2019 · a neutral, or stable, atom is composed of an equal amount of three components: Luckily, one electron has the same charge (with opposite sign) as a proton.. Luckily, one electron has the same charge (with opposite sign) as a proton.

Jul 04, 2012 · a neutral atom is an atom where the charges of the electrons and the protons balance. The amount of charge in a single proton is equal to the amount of charge possessed by a single electron. Protons are positively charged particles, electrons are negatively charged and neutrons are neutral. Here we have, actually, three questions compressed into one:.. Electrons are negatively charged, ptotons are positively charged and neutrons don't have ant charge.

Why an atom doesn't have electr... Protons are positively charged particles, electrons are negatively charged and neutrons are neutral. Mar 30, 2020 · atoms are neutral because they have same number of positively charged particles, protons, and negatively charged particles, electrons. Nov 25, 2020 · an atom is electrically neutral because the overall charge of an atom is zero. The atomic number is 6 since there are 6 protons. The atoms are made of three subatomic particles called protons, electrons and neutrons. What is a neutral atom example? Jul 04, 2012 · a neutral atom is an atom where the charges of the electrons and the protons balance. Why an atom doesn't have a net (monopole) electric charge?
Protons have a positive electrical charge, neutrons are neutral, and electrons have a negative charge... Atoms are usually neutral because they have the same number of protons as they do electrons. They contain the same number of protons as electrons.by definition, an ion is an electrically charged particle produced by either removing electrons from a neutral atom to give a positive ion or adding electrons to a neutral atom to give a negative ion. Luckily, one electron has the same charge (with opposite sign) as a proton. Why an atom doesn't have a net (monopole) electric charge? Jul 04, 2012 · a neutral atom is an atom where the charges of the electrons and the protons balance. Luckily, one electron has the same charge (with opposite sign) as a proton. Atoms also have another particle type in the nucleus called neutrons that have no charge. Why an atom doesn't have electr.

Protons are positively charged particles, electrons are negatively charged and neutrons are neutral. Protons have a positive electrical charge, neutrons are neutral, and electrons have a negative charge. A neutral atom is an atom where the charges of the electrons and the protons balance.

Neutral atoms can be turned into positively charged ions by. The atoms are made of three subatomic particles called protons, electrons and neutrons. Protons are positively charged particles, electrons are negatively charged and neutrons are neutral. What is a neutral atom example?

Why an atom doesn't have a net (monopole) electric charge? The amount of charge in a single proton is equal to the amount of charge possessed by a single electron. Jul 04, 2012 · a neutral atom is an atom where the charges of the electrons and the protons balance. Why an atom doesn't have a net (monopole) electric charge? The atomic number is 6 since there are 6 protons. A neutral atom is an atom where the charges of the electrons and the protons balance. Mar 30, 2020 · atoms are neutral because they have same number of positively charged particles, protons, and negatively charged particles, electrons. The overall charge of an atom is zero because the atoms have an equal number. Neutral atoms can be turned into positively charged ions by. Atoms also have another particle type in the nucleus called neutrons that have no charge. Why an atom doesn't have electr. Luckily, one electron has the same charge (with opposite sign) as a proton.